Intensive variables don’t change with size or situation
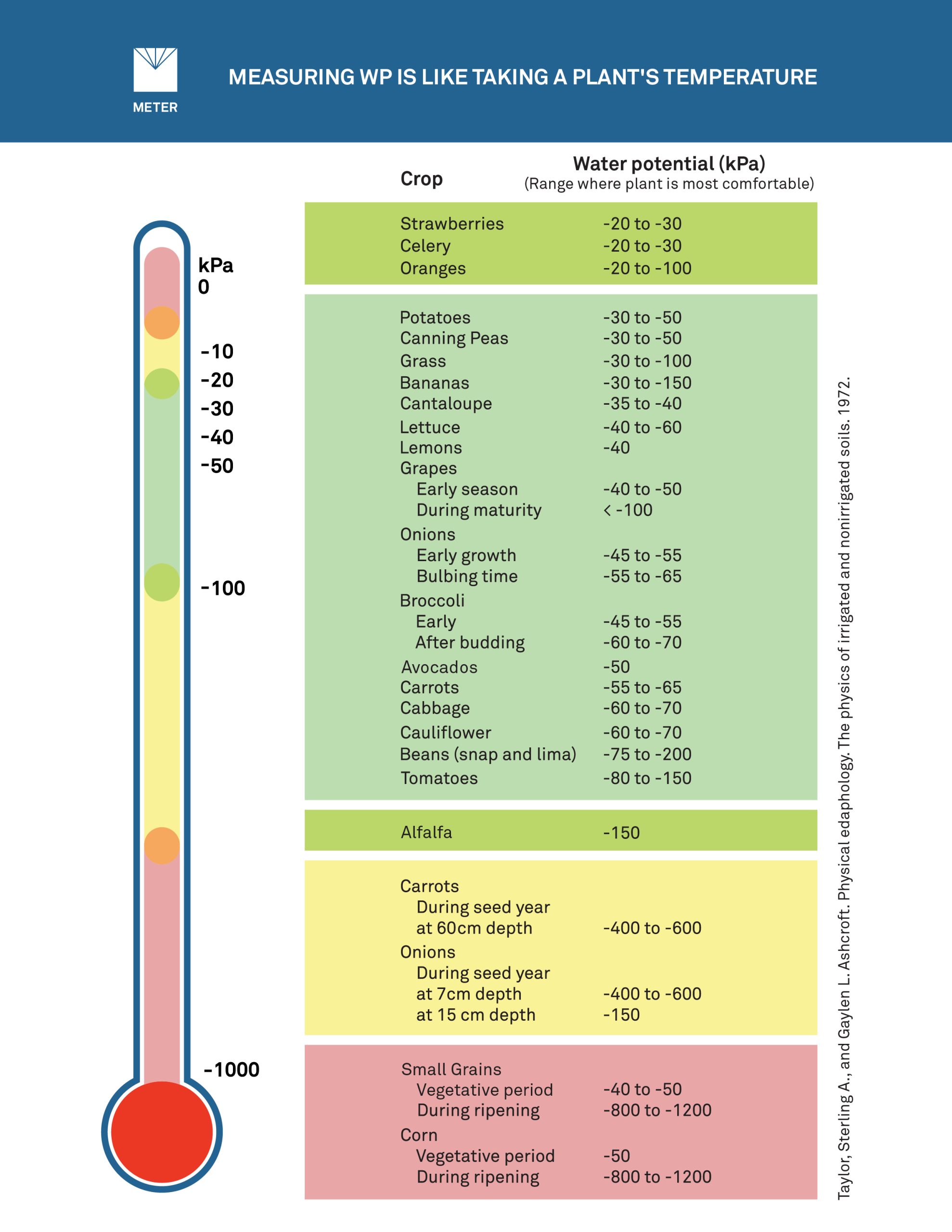
Water potential is the energy required, per quantity of water, to transport an infinitesimal quantity of water from the sample to a reference pool of pure free water. Water potential is often compared to temperature. Both are considered “intensive” variables that describe the intensity or quality of matter or energy. For example, the thermal state of a substance can be described in terms of both heat content and temperature. Temperature is an obvious way to define the intensity of comfort in a human being.
Heat content doesn’t define comfort because heat content at a specific comfort level will be higher in a large room and lower in a small room. The temperature measurement defines comfort without any other variables entering into the equation.
Water content doesn’t predict how water moves
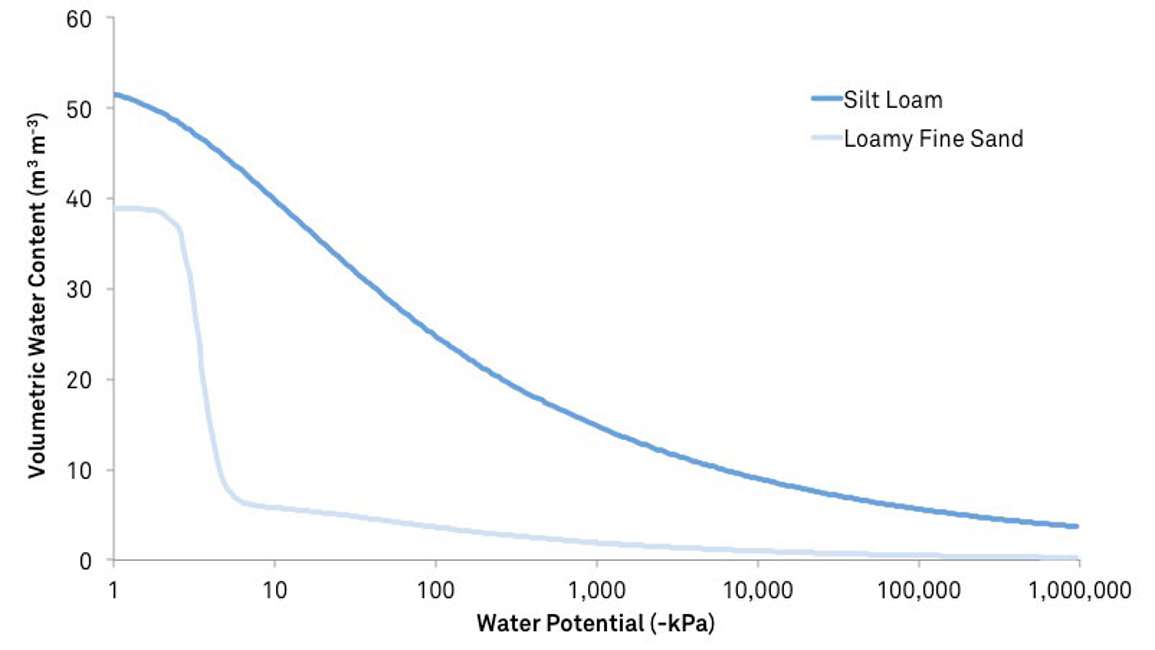
Similar to heat content, water content is an amount. It’s an extensive variable. It changes with size and situation. Consider the following paradoxes:
- A soil with fairly low volumetric water content can have plenty of plant-available water, and a soil with high water content can have almost none
- Gravity pulls water down through the profile, but water moves up into the soil from a water table
- Two adjacent patches of soil at equilibrium can have significantly different water content
In these and many other cases, water content data are confusing because they don’t predict how water moves. Water potential measures the energy state of water and thus explains realities of water movement that otherwise defy intuition. Like temperature, water potential defines the comfort level of a plant. Similar to the room-size analogy for temperature, if the water potential is known, it’s possible to predict whether plants will grow well or be stressed in any environment.
Learn more about intensive vs. extensive variables in this chalk talk by soil physicist, Dr. Colin Campbell.